| Mike's Pages |
Links |
Hydrogen Adsorption on Rh, Ni, and Pd Functionalized Single-Walled Boron Nitride Nanotubes
L. P. Zhang, P. Wu, and M. B. Sullivan
Institute of High Performance Computing, 1 Fusionopolis Way, #16-16 Connexis, Singapore 138632
Publication Date (Web): February 23, 2011
J. Phys. Chem. C, 2011, 115 4289-4296.
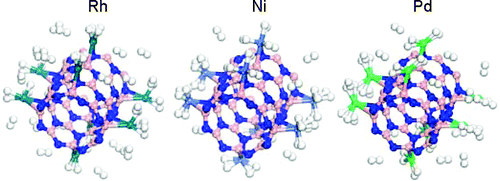
ABSTRACT:
Rhodium, nickel, and palladium functionalized single-walled boron nitride nanotubes (SWBNNTs) and their applications to hydrogen storage have been investigated using density functional theory (DFT). Single Rh, Ni, and Pd atoms prefer to bind strongly at the axial bridge site of BN nanotube, and each Rh, Ni and Pd atom bound on BNNT may adsorb up to four, three, and two H2 molecules, respectively, with the H−H bonds of H2 molecules significantly elongated. More H2 molecules would bind with metal atoms and tubes when four metal atoms are dispersed at the bridge sites per cell, the presence of Rh, Ni, and Pd metal atoms leads to high hydrogen storage capacity on BNNTs. In addition, our calculation results also show that the nature of interaction between hydrogen and metal-doped BNNT is due to the hybridization of the metal d orbital with the hydrogen s orbital. Our work not only predicts hydrogen capacities and their binding energies for metal-doped BNNTs but also advances the understanding of the nature of hydrogen adsorption for efficient hydrogen storage.
DOI: 10.1021/jp1078554